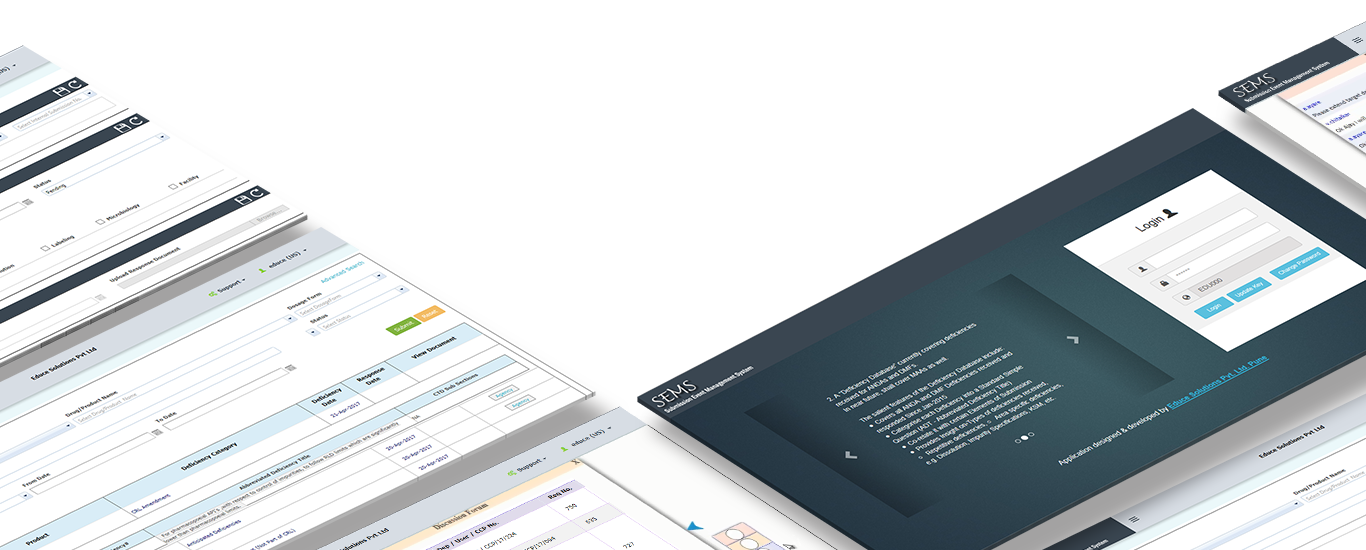
Submission Event Management Software (SEMS)
SEMS is software solution which tracks and manages main events for Regulatory Submission post initial Dossier Submission.

Key Features:
- Elaborate management of Queries from Regulatory authorities.
- Objective of the Software is to reduce Queries for future Drug Products by having detail check list of issues to be taken care during Product development.
- Provision to enter historical queries and related responses.
- Provision to define Abbreviated queries based on the communication received from regulatory authorities.
- Provision to assign each task to different department users to address the relevant queries.
- Emails, discussion forum and provision to document all communications across departments while addressing queries.
- Software serves as knowledge base for future drug development.
- Provision to load relevant guidelines to enable relevant departments while addressing queries.