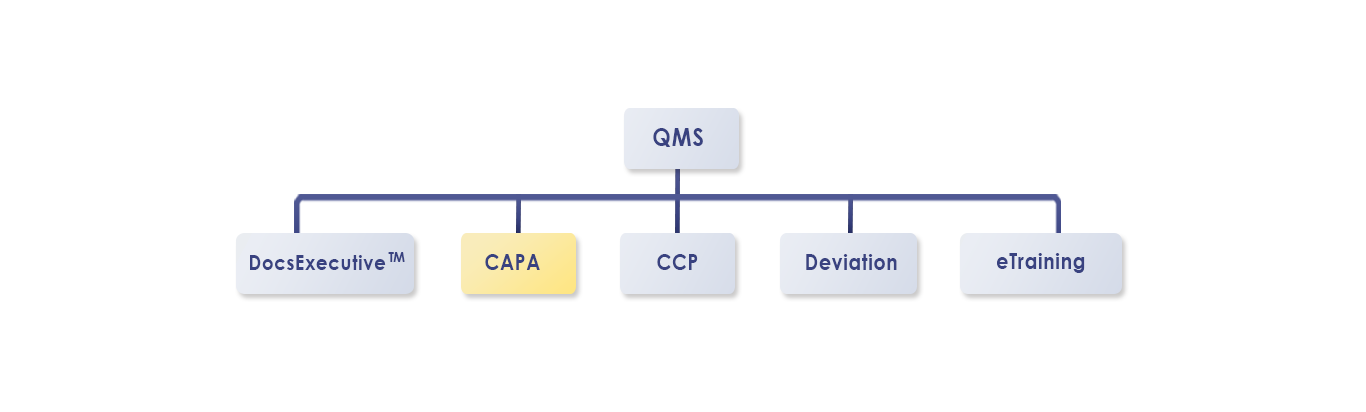
Key Features:
- It manages the complete flow from initiation of CAPA till closure after effective review.
- Architecture allows application access using web technology .
- Electronic Signatures and audit trails as per 21 CFR Part 11 guidelines of USFDA.
- Standard questionnaire can be configured which is to be answered by concerned departments during the CAPA flow.
- Impacted departments can add Corrective and Preventive actions as suggested by Initiating department and Site QA.
- To verify effectiveness of CAPA each action can be assigned to different reviewers. Input of effectiveness check is recorded.
- Auto task reminders through emails and notifications based on target dates given for action points and other activities like review/approve/impact analysis.
- CAPA can be monitored and tracked by Site QA using different tracking options.
CAPA Flowchart:
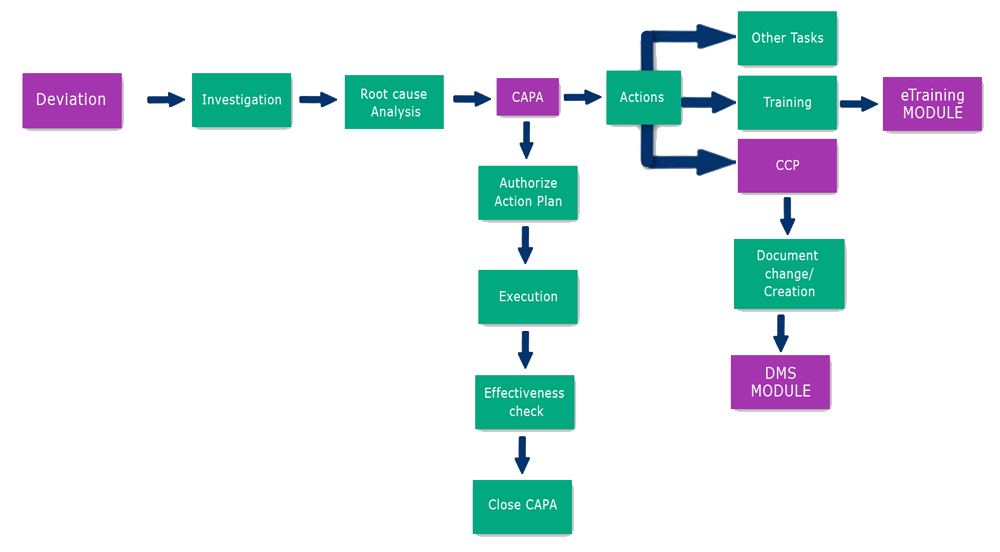
-->
-->
-->