Key Features:
- It manages the complete flow from initiating deviation, conducting investigation, root cause analyses and generating CAPA if required, till closure.
- Planned and unplanned deviations can be managed.
- Architecture allows application access using web technology.
- Electronic Signatures and audit trails as per 21 CFR Part 11 guidelines of USFDA.
- Primary and secondary investigation can be carried out during the deviation work flow depending on Site QA requirement.
- Root cause analysis, Impact assessment categorization based on which MIS reports can be generated.
- Auto task reminders through emails and notifications based on target dates given for action points and other activities like review/approve/impact analysis.
- Deviation can be monitored and tracked by Site QA using different tracking options.
Deviation Flowchart:
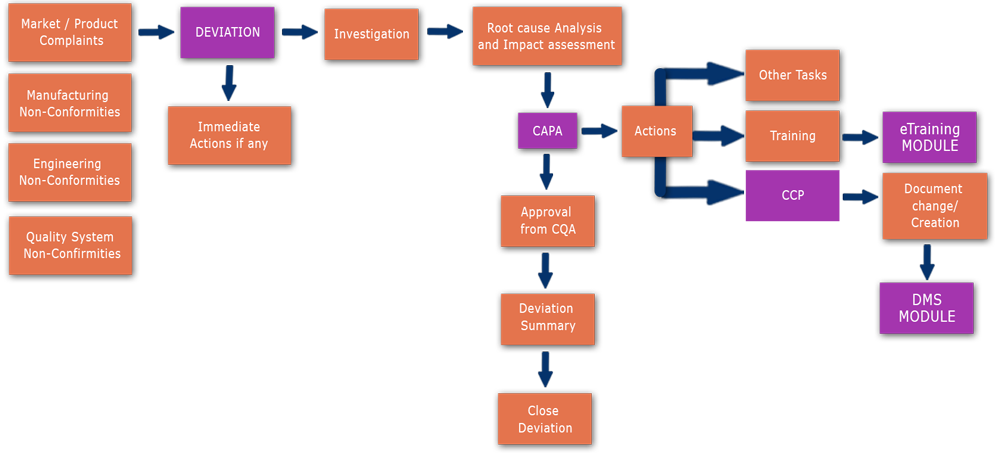
-->
-->
-->